Steel
Steel is an alloy of iron and other elements, primarily carbon, widely used in construction and other applications because of its high tensile strength and low cost. The base metal, iron, is able to take on two crystalline forms (allotropic forms), body centered cubic (BCC) and face centered cubic (FCC), depending on its temperature. It is the interaction of those allotropes with the alloying elements, primarily carbon, that gives steel and cast iron their range of unique properties. In the body-centred cubic arrangement, there is an additional iron atom in the centre of each cube, and in the face-centred cubic, there is one at the center of each of the six faces of the cube. Carbon, other elements, and inclusions within iron act as hardening agents that prevent the movement of dislocations that otherwise occur in the crystal lattices of iron atoms.
The carbon in typical steel alloys may contribute up to 2.1% of its weight. Varying the amount of alloying elements, their presence in the steel either as solute elements, or as precipitated phases, retards the movement of those dislocations that make iron comparatively ductile and weak, and thus controls its qualities such as the hardness, ductility, and tensile strength of the resulting steel. Steel's strength compared to pure iron is only possible at the expense of iron's ductility, of which iron has an excess.
Although steel had been produced in bloomery furnaces for thousands of years, steel's use expanded extensively after more efficient production methods were devised in the 17th century with the production of blister steel and then crucible steel. With the invention of theBessemer process in the mid-19th century, a new era of mass-produced steel began. This was followed by Siemens-Martin process and then Gilchrist-Thomas process that refined the quality of steel. With their introductions, mild steel replaced wrought iron.
Further refinements in the process, such as basic oxygen steelmaking (BOS), largely replaced earlier methods by further lowering the cost of production and increasing the quality of the product. Today, steel is one of the most common materials in the world, with more than 1.3 billion tons produced annually. It is a major component in buildings, infrastructure, tools, ships, automobiles, machines, appliances, and weapons. Modern steel is generally identified by various grades defined by assorted standards organizations.
Material properties
Iron is commonly found in the Earth's crust in the form of an ore, usually an iron oxide, such as magnetite,hematite etc. Iron is extracted from iron ore by removing the oxygen through combination with a preferred chemical partner such as carbon that is lost to the atmosphere as carbon dioxide. This process, known assmelting, was first applied to metals with lower melting points, such as tin, which melts at about 250 °C (482 °F) and copper, which melts at about 1,100 °C (2,010 °F) and the combination, bronze, which is liquid at less than 1,083 °C (1,981 °F). In comparison, cast iron melts at about 1,375 °C (2,507 °F).Small quantities of iron were smelted in ancient times, in the solid state, by heating the ore buried in acharcoal fire and welding the clumps together with a hammer, squeezing out the impurities. With care, the carbon content could be controlled by moving it around in the fire.
All of these temperatures could be reached with ancient methods that have been used since the Bronze Age. Since the oxidation rate of iron increases rapidly beyond 800 °C (1,470 °F), it is important that smelting take place in a low-oxygen environment. Unlike copper and tin, liquid or solid iron dissolves carbon quite readily. Smelting, using carbon to reduce iron oxides, results in an alloy (pig iron) that retains too much carbon to be called steel. The excess carbon and other impurities are removed in a subsequent step.
Other materials are often added to the iron/carbon mixture to produce steel with desired properties. Nickel and manganese in steel add to its tensile strength and make the austenite form of the iron-carbon solution more stable, chromium increases hardness and melting temperature, and vanadium also increases hardness while making it less prone to metal fatigue.
To inhibit corrosion, at least 11% chromium is added to steel so that a hard oxide forms on the metal surface; this is known as stainless steel. Tungsten interferes with the formation of cementite, allowing martensite to preferentially form at slower quench rates, resulting in high speed steel. On the other hand, sulfur, nitrogen, and phosphorus make steel more brittle, so these commonly found elements must be removed from the steel melt during processing.
The density of steel varies based on the alloying constituents but usually ranges between 7,750 and 8,050 kg/m3 (484 and 503 lb/cu ft), or 7.75 and 8.05 g/cm3 (4.48 and 4.65 oz/cu in).
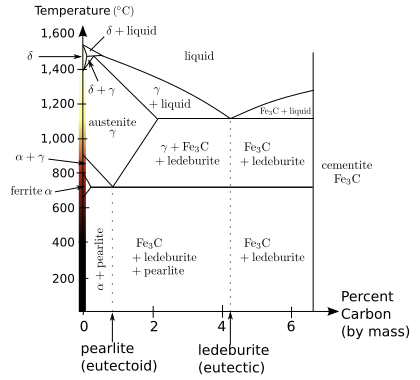
Even in a narrow range of concentrations of mixtures of carbon and iron that make a steel, a number of different metallurgical structures, with very different properties can form. Understanding such properties is essential to making quality steel. At room temperature, the most stable form of pure iron is the body-centered cubic (BCC) structure called alpha iron or α-iron. It is a fairly soft metal that can dissolve only a small concentration of carbon, no more than 0.005% at 0 °C (32 °F) and 0.021 wt% at 723 °C (1,333 °F). The inclusion of carbon in alpha iron is called ferrite. At 910 °C pure iron transforms into a face-centered cubic (FCC) structure, called gamma iron or γ-iron. The inclusion of carbon in gamma iron is called austenite. The FCC structure of austenite can dissolve considerably more carbon, as much as 2.1% (38 times that of ferrite) carbon at 1,148 °C (2,098 °F), which reflects the upper carbon content of steel, beyond which is cast iron.[7]When carbon moves out of solution with iron it forms a very hard, but brittle material called cementite (Fe3C).
When steels with exactly 0.8% carbon (known as a eutectoid steel), are cooled, the austenitic phase (FCC) of the mixture attempts to revert to the ferrite phase (BCC). The carbon no longer fits within the FCC austenite structure, resulting in an excess of carbon. One way for carbon to leave the austenite is for it to precipitate out of solution ascementite, leaving behind a surrounding phase of BCC iron called ferrite that is able to hold the carbon in solution. The two, ferrite and cementite, precipitate simultaneously producing a layered structure called pearlite, named for its resemblance to mother of pearl. In a hypereutectoid composition (greater than 0.8% carbon), the carbon will first precipitate out as large inclusions of cementite at the austenite grain boundaries and then when the composition left behind is eutectoid, the pearlite structure forms. For steels that have less than than 0.8% carbon (hypoeutectoid), ferrite will first form until the remaining composition is 0.8% at which point the pearlite structure will form. No large inclusions of cementite will form at the boundaries.The above assumes that the cooling process is very slow, allowing enough time for the carbon to migrate.
As the rate of cooling is increased the carbon will have less time to migrate to form carbide at the grain boundaries but will have increasingly large amounts of pearlite of a finer and finer structure within the grains; hence the carbide is more widely dispersed and acts to prevent slip of defects within those grains, resulting in hardening of the steel. At the very high cooling rates produced by quenching, the carbon has no time to migrate but is locked within the face center austenite and forms martensite. Martensite is highly strained and stressed supersaturated form of carbon and iron and is exceedingly hard but brittle. Depending on the carbon content, the martensitic phase takes different forms. Below 0.2% carbon, it takes on a ferrite BCC crystal form, but at higher carbon content it takes a body-centered tetragonal (BCT) structure. There is no thermal activation energyfor the transformation from austenite to martensite. Moreover, there is no compositional change so the atoms generally retain their same neighbors.
Martensite has a lower density (it expands) than does austenite, so that the transformation between them results in a change of volume. In this case, expansion occurs. Internal stresses from this expansion generally take the form of compression on the crystals of martensite and tension on the remaining ferrite, with a fair amount of shear on both constituents. If quenching is done improperly, the internal stresses can cause a part to shatter as it cools. At the very least, they cause internal work hardening and other microscopic imperfections. It is common for quench cracks to form when steel is water quenched, although they may not always be visible.

No comments:
Post a Comment